DrNaka
Founding Member
- Joined
- Mar 1, 2011
- Messages
- 78
- Reaction score
- 3
I do not know how the education in other countries are but in Japan we have chemistry lecture in middle or high school (I forgot which because it was about half century ago) about reaction of metal with acid.
A piece of iron or aluminum is reacted with concentrated or diluted hydrochloric, nitric and sulfuric acid.
At middle or high school level the schoolchild will find out that the piece of iron or aluminum does not react with concentrated nitric acid (68%) and the teacher will explain that it is passivation. The concentrated nitric acid works here as oxidant and makes a thin layer of insoluble metal oxide at the surface. So the underneath metal cannot react with the acid anymore.
If you go to University and learn a bit about corrosion you will have a lecture about Pourbaix diagram.
Here is the Wiki about it:
http://en.wikipedia.org/wiki/Pourbaix_diagram
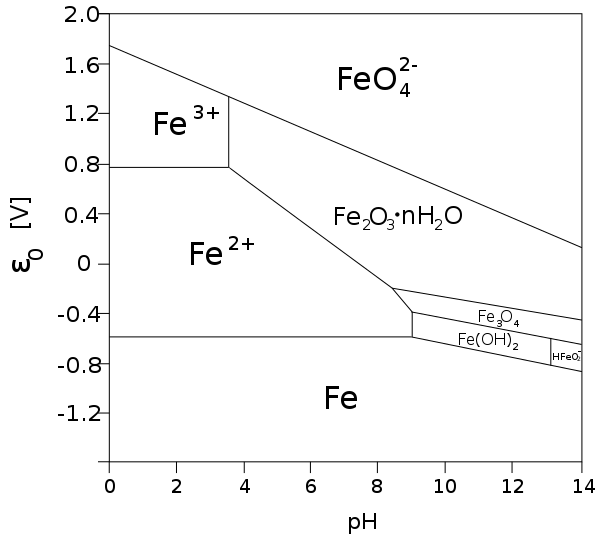
The region of Fe2O3 nH2O is the region where iron does get passivated.
This is about corrosion in water at room temperature.
But how about SS?
Chrome and nickel in the alloy help to make the Fe2O3 nH2O region get bigger. Also it helps the alloy to get a oxidated surface in the air.
These passivation are not visible to the human eye.
A SS knife will have similar metallic shine as a carbon knife though the SS has a oxidation layer.
Continued to next part....
A piece of iron or aluminum is reacted with concentrated or diluted hydrochloric, nitric and sulfuric acid.
At middle or high school level the schoolchild will find out that the piece of iron or aluminum does not react with concentrated nitric acid (68%) and the teacher will explain that it is passivation. The concentrated nitric acid works here as oxidant and makes a thin layer of insoluble metal oxide at the surface. So the underneath metal cannot react with the acid anymore.
If you go to University and learn a bit about corrosion you will have a lecture about Pourbaix diagram.
Here is the Wiki about it:
http://en.wikipedia.org/wiki/Pourbaix_diagram

The region of Fe2O3 nH2O is the region where iron does get passivated.
This is about corrosion in water at room temperature.
But how about SS?
Chrome and nickel in the alloy help to make the Fe2O3 nH2O region get bigger. Also it helps the alloy to get a oxidated surface in the air.
These passivation are not visible to the human eye.
A SS knife will have similar metallic shine as a carbon knife though the SS has a oxidation layer.
Continued to next part....




